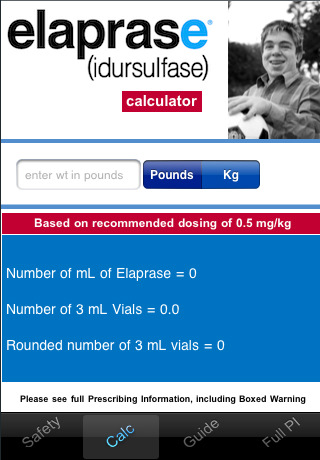
Number of vials of ELAPRASE to be used for a patient is based on patient weight & the recommended dose.This free app allows healthcare professionals to quickly enter patient's weight to find the total number of mL's of ELAPRASE to be administered & the corresponding number of ELAPRASE vials necessary for the required dose� Indication
ELAPRASE (idursulfase) is indicated for patients with Hunter syndrome (Mucopolysaccharidosis II,MPS II).ELAPRASE has been shown to improve walking capacity in these patients
Important Safety Considerations
WARNING Risk of anaphylactic reactions: Life-threatening anaphylactic reactions have been observed in some patients during ELAPRASE infusions.Therefore,appropriate medical support should be readily available when ELAPRASE is administered.Biphasic anaphylactic reactions have also been observed after ELAPRASE administration and patients who have experienced anaphylactic reactions may require prolonged observation.Patients with compromised respiratory function or acute respiratory disease may be at risk of serious acute exacerbation of their respiratory compromise due to infusion reactions,and require additional monitoring.
-Life-threatening anaphylactic reactions have been observed in some patients during ELAPRASE infusions.Reactions have included respiratory distress,hypoxia,hypotension,seizure,loss of consciousness,urticaria and/or angioedema of the throat & tongue.Biphasic anaphylactic reactions have also been reported to occur after administration of ELAPRASE approximately 24 hours after treatment & recovery from an initial anaphylactic reaction that occurred during ELAPRASE infusion.Interventions for biphasic reactions have included hospitalization,& treatment with epinephrine,inhaled beta-adrenergic agonists & corticosteroids
-Patients with compromised respiratory function or acute respiratory disease may be at higher risk of life- threatening complications from infusion reactions.Consider delaying the ELAPRASE infusion in patients with concomitant acute respiratory and/or febrile illness
-Because of the potential for severe infusion reactions,appropriate medical support should be readily available when ELAPRASE is administered.Because of the potential for biphasic anaphylactic reactions after ELAPRASE administration,patients who experience initial severe or refractory reactions may require prolonged observation.If a severe infusion reaction occurs,immediately suspend the infusion of ELAPRASE & initiate appropriate treatment.When severe infusion reactions occurred during clinical studies,subsequent infusions were managed by use of antihistamines and/or corticosteroids prior to or during infusions,a slower rate of ELAPRASE administration and/or early discontinuation of the ELAPRASE infusion if serious symptoms developed.With these measures,no patient discontinued treatment permanently due to an allergic reaction
-In clinical studies,51% of patients treated with ELAPRASE developed anti-idursulfase IgG antibodies.These patients had an increased incidence of infusion reactions,including allergic reactions.The relationship between the presence of anti-idursulfase antibodies & clinical efficacy outcomes is unknown.
-In clinical studies,the most common adverse events seen in patients treated with ELAPRASE were pyrexia (63%),headache (59%),& arthralgia (31%).The most common adverse reactions requiring intervention were infusion- related reactions including: headache,fever,cutaneous reactions (rash,pruritus,erythema & urticaria) & hypertension
-The most frequent serious adverse events related to the use of ELAPRASE were hypoxic episodes.Other notable serious adverse reactions that occurred in ELAPRASE patients but not in placebo patients included one case each of cardiac arrhythmia,pulmonary embolism,cyanosis,respiratory failure,infection & arthralgia
Please see full Prescribing Information(http://www.elaprase.com/pdf/ElaprasePI40-0120_REV_0_GT4.pdf) including boxed warning
less
|