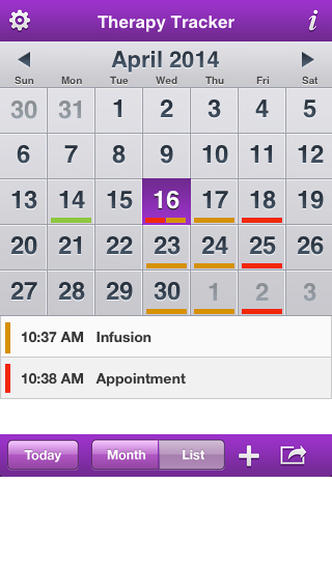
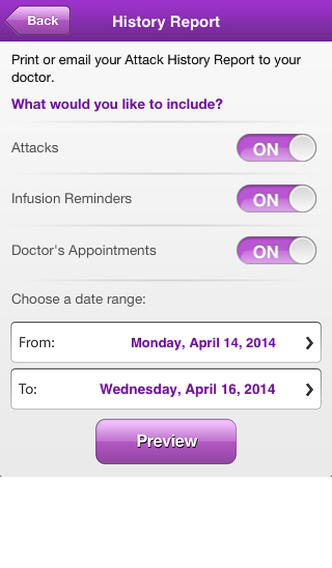
KEEP YOUR HAE THERAPY ON TRACK with the HAE Mobile Therapy Tracker. This tool is for adults & teenagers with hereditary angioedema & who use CINRYZE® (C1 esterase inhibitor [human]) therapy for routine prevention of HAE attacks. This app allows you to schedule infusion and Dr. appointment reminders, track your history of HAE attacks & email/print your progress to share.
This app is intended for U.S. residents only and should not replace normal record keeping or professional medical advice. Speak to your healthcare provider with any questions or concerns. Take CINRYZE as prescribed by your doctor.
See Full Prescribing Information at: http://www.cinryze.com/pdfs/cinryze-prescribing-information.pdf.
Comments posted on iTunes are not associated with, nor endorsed by Shire.
INDICATION
CINRYZE® (C1 esterase inhibitor [human]) is an injectable prescription medicine that is used to help prevent swelling and/or painful attacks in teenagers and adults with Hereditary Angioedema (HAE).
IMPORTANT SAFETY INFORMATION
You should not use CINRYZE if you have had life-threatening immediate hypersensitivity reactions, including anaphylaxis to the product.
Tell your healthcare provider about all of your medical conditions, including if you:
•have an indwelling catheter/access device in one of your veins.
•have a history of blood clots, heart disease, or stroke.
•are taking birth control pills or androgens.
•are pregnant or planning to become pregnant. It is not known if CINRYZE can harm your unborn baby.
•are breastfeeding or plan to breastfeed. It is not known if CINRYZE passes into your milk and if it can harm your baby.
Tell your healthcare provider and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines such as over-the-counter medicines, supplements, or herbal remedies.
Allergic reactions may occur with CINRYZE. Call your healthcare provider or get emergency support services right away if you have any of the following symptoms:
•wheezing
•difficulty breathing
•chest tightness
•turning blue (look at lips and gums)
•fast heartbeat
•swelling of the face
•faintness
•rash
•hives
Serious blood clots may occur with CINRYZE. Call your healthcare provider or get emergency support services right away if you have any of the following symptoms:
•pain and/or swelling of an arm or leg with warmth over the affected area
•discoloration of an arm or leg
•unexplained shortness of breath
•chest pain or discomfort that worsens on deep breathing
•unexplained rapid heart rate
•numbness or weakness on one side of the body
Because CINRYZE is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
The most common side effects seen with CINRYZE were headache, nausea, rash, and vomiting. These are not all the possible side effects of CINRYZE. Tell your healthcare provider about any side effect that bothers you or that does not go away. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088. You can also report side effects to ViroPharma Medical Information at (866) 331-5637.
Medicines are sometimes prescribed for purposes other than those listed here. Do not use CINRYZE for a condition for which it is not prescribed. Do not share CINRYZE with other people, even if they have the same symptoms that you have.
Before starting CINRYZE, please read the Patient Information carefully and each time you get a refill. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about CINRYZE. If you have questions after reading this, ask your healthcare provider.
Please read the full prescribing information.
Approved Information as of March 2014.
CINRYZE and the associated logos are trademarks of Shire or its affiliates.
US/CIN-00108/14 05/2014
less